Excessive overpressure can occur in almost all production areas and processes in the pharmaceutical industry. If the pressure exceeds or falls below the limits defined for the process, serious damage can occur to the respective plant components. Possible consequences include production stoppages and significant economic damage. Therefore, in situations where the pressure exceeds or undershoots the permissible operating range, a reliable and fast-acting pressure-relief device is essential.
Many operators in the pharmaceutical industry already use REMBE high-performance rupture discs in their processing plants – because the advantages of these pressure-relief devices ensure permanently safe operation:
- Thanks to their leak-tightness, our rupture discs prevent the escape of process media
- The complete cross-sectional release of the burst element provides immediate pressure relief for the system
- REMBE rupture discs have a response time of mere milliseconds
In order to customise the design of our rupture discs for each customer, numerous factors must be considered ahead of time. Reliable and economical rupture discs are not standardised products. Instead, they are individually adapted to the respective process conditions, designed accordingly and then manufactured specifically for the customer’s applications. Numerous parameters are taken into account to ensure optimal performance.
These parameters include:
- the medium in the system to be protected (liquid, sticky, gaseous, etc.)
- the operating temperature for which the rupture disc is designed (room or max. operating temperature).
- the required resistance to vacuum
- the mounting method (tri-clamp or holder system)
- the required pressure-relief area
- and many more
As well as pipelines, in the pharmaceutical industry there are additional plant elements, such as reactors, filling plants and tank farms, which must be protected against overpressure and vacuum and equipped with safety-related components. However, protection against impermissible pressures is not the only consideration in the pharmaceutical industry. Leak-tightness and the sterility of the respective components are also critical factors. Last but not least, reducing downtime is a key requirement with regard to the use of rupture discs.
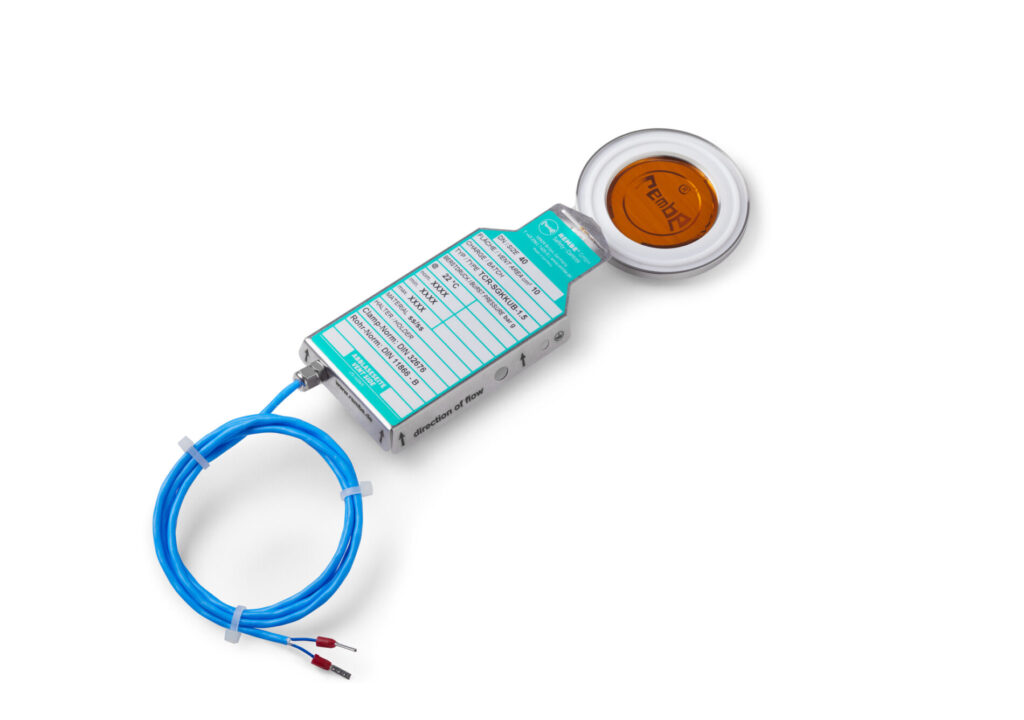
In order to keep costly system downtime to a minimum, it is essential that the rupture discs can be installed quickly and easily. Our rupture discs can be installed using a tri-clamp connection, which provides a sterile and extremely reliable seal. When REMBE developed the KUB clean reverse acting buckling pin rupture disc, one of our goals was to meet the requirements for sensitive processes in the pharmaceutical industry, such as leak tightness and sterility. This reverse acting disc is installed with the bulge facing the process and allows the operator to achieve an operating pressure ratio of up to 98%. Overall, this reverse acting rupture disc is an extremely durable and robust solution that virtually eliminates the risk of premature bursting.

The unique design of the KUB clean is based on a two-layer structure, whereby the side facing the process has a completely smooth surface, which prevents product build-up. The integrated seal ensures the required degree of tightness for the rupture disc. It meets all hygienic design requirements (FDA and USP Class VI compliant) and is suitable for both CIP and SIP. In addition, the seals can be replaced; the rupture disc can thus be reused immediately after an inspection or cleaning.
Each KUB can be optionally equipped with a signalling function. As soon as an excessive overpressure is reached and the rupture disc has burst, signalling becomes a very important safety factor in sensitive production facilities by ensuring that the rupture disc is subject to permanent monitoring.