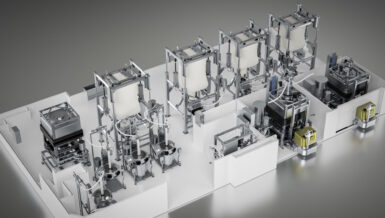
This joint project demonstrates the transformative potential of purpose-built equipment when paired with forward-thinking pharmaceutical science. The outcome? A scalable solution for aseptic manufacturing that is cleanroom-compatible, regulation-compliant, and adaptable to future market demands.
Meeting the Moment: A Need for Capacity and Compliance
Founded in 1974 at the University of Iowa, UI Pharmaceuticals is the largest university-affiliated FDA-registered drug product CDMO in the United States. The organization supports over 80–100 clients annually, producing an average of 100 product batches each year across a wide range of dosage forms—including sterile liquids and lyophilized products, tablets, capsules, gels, and more.
With a portfolio that includes potent compounds, peptides, and small molecules across preclinical to commercial stages, UI Pharmaceuticals is uniquely positioned to support biotech, pharma, and academic clients with both agility and rigor. However, as demand for aseptically manufactured products grew, the limitations of semi-automatic vial-filling lines became apparent.
The organization needed to scale its sterile manufacturing capabilities while meeting stringent FDA and EMA requirements. That meant building a highly flexible, automated solution that could support both clinical trial production and eventual commercial runs—all within the spatial and operational constraints of an existing facility.
Enter Integrated Containment Systems: Engineering a Turnkey Solution
UI Pharmaceuticals turned to Integrated Containment Systems, an American manufacturer with decades of experience in cleanroom-compatible equipment, precision dosing, and containment system integration. ICS specializes in designing and building customized solutions for powder handling, isolators, blending systems, and aseptic fill-finish equipment.

For this project, ICS delivered two mirror-image, fully automated sterile fill-finish lines. Each line supports the aseptic processing of liquid and lyophilized sterile drug products in glass[LS1] vials ranging from 5 mL to 100 mL. The system is the most advanced and flexible fill-finish installations ever undertaken by an academic institution.
Key design goals included:
- Automation & Efficiency: Replace a low-capacity, manual cleanroom system with a fully automated, high-throughput solution capable of processing thousands of vials per day.
- Configurability: Enable 14 different vial path configurations across two independent production lines—offering exceptional batch flexibility.
- Containment: Ensure product and personnel safety with fully enclosed isolator gloveboxes, maintaining ISO 5 cleanroom conditions.
- Compliance: Incorporate a validated SCADA system aligned with 21 CFR Part 11 for electronic records and signatures.
- Integration: Seamlessly synchronize equipment from multiple OEMs into one cohesive, FDA-compliant system.
From Concept to Completion: Collaboration in Action
ICS served as the single point of contact throughout the entire equipment lifecycle—handling:
- Requirements definition
- Custom design and layout
- Equipment procurement, fabrication, assembly and site supervision.
- SCADA control system integration
- On-site installation and commissioning
- FDA- and EMA-ready validation documentation
Their engineering team worked closely with UI Pharmaceuticals and site contractors to adapt the solution to the physical space and operational workflow, reducing future retrofitting needs and enabling fast-track implementation.
Each production line integrates six major pieces of equipment, five subsystems, and various automated interfaces to perform vial washing, depyrogenation, liquid filling, lyophilization, and capping. The system’s ability to run Line 1, Line 2, or both simultaneously gives UI Pharmaceuticals unmatched operational flexibility.
Most importantly, the isolator-based design eliminates the need for cleanroom garbing and reduces operator intervention, thereby minimizing contamination risks and ensuring reproducible sterility assurance levels (SAL).
The Impact: A Leap Forward in Pharma Manufacturing
Thanks to ICS’s deep expertise in cGMP, aseptic process design, and complex system integration, UI Pharmaceuticals now operates one of the most agile, compliant, and production-ready fill-finish lines in the academic pharmaceutical sector. The line supports:
- Batch sizes up to 200 L
- Sterile products for clinical trials and commercial supply
- Rapid scale-up of novel formulations
- Material handling for high-potency and cytotoxic compounds
The partnership proved especially valuable during the validation phase, as ICS’s integrated approach eliminated the need for an external validation vendor—saving significant time and cost.
This installation is now a benchmark for other institutions and CDMOs seeking scalable, high-compliance solutions in sterile manufacturing. For the pharmaceutical clients of UI Pharmaceuticals, it means faster timelines, greater batch flexibility, and increased confidence in product quality.
A Model for Industry Collaboration
This project exemplifies what’s possible when a strategic CDMO and an experienced equipment manufacturer work as true partners from the ground up.
ICS didn’t just sell equipment—they engineered a custom solution aligned with UI Pharmaceuticals’ unique mission: supporting innovation, research, and life-saving therapies from concept to market.
About Integrated Containment Systems (ICS)/Custom Powder Systems(CPS)
Based in Springfield, Missouri, Custom Powder Systems designs and builds containment and powder-handling systems that meet the highest standards for safety, efficiency, and compliance. Their turnkey approach includes design, fabrication, automation, and validation. Learn more at https://custom-powder.com.
About UI Pharmaceuticals
UI Pharmaceuticals is the longest-running, university-affiliated, FDA-registered pharmaceutical manufacturing facility in the United States. They provide end-to-end drug product services—from pre-formulation through commercial production—with a focus on quality, safety, and compliance. Learn more at https://www.medicine.uiowa.edu/uipharm.
[LS1]We can fill both molded and tubular vials