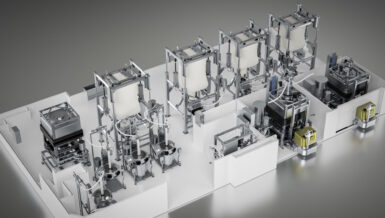
It has systematically developed its range of services and process partnerships, and structured itself so that this new philosophy is driven from the very top of its organization. Contrary to its days as an engineering-based company solely focused on developing technologically advanced pharmaceutical equipment, it is now entrenching itself in its client’s early-stage development processes. The goal of its North Star – “Together – from Lab to Production” – is to bolster those processes and guarantee success as oral solid dosage (OSD) products move throughout the entire lifecycle from formulation and process development, to scale-up, production, and later process optimization.
A Dynamic Portfolio
Employing a Quality by Design approach, Fette Compacting’s process consultants will examine a client’s critical material attributes (CMAs) and link them to relevant critical process parameters (CPPs). The result is an optimal level of tablet quality, intended to endure for many years. The company’s strength lies in its ability to relate powder properties to the compression process and to take a holistic view of any related challenges. It now boasts an expanded equipment portfolio whose depth matches that of the new organizational structure. The F Lab 10, for example, is a desktop powder compaction analyzer that allows for the analysis of powder compression behavior prior to more advanced stages of process development.

Supporting process analysis further still, Fette Compacting can mimic process steps using specially designed emulators. Unlike traditional simulators, which require conversion steps and are essentially “virtual”, emulators truly replicate real production operations. Their utilization can accelerate process development by a significant degree; only small amounts of material are required for analysis, while still resulting in accurate predictions.
In the long term, the client is empowered to validate their formulations and process parameters early and rectify issues in the development phase. Overall, the ultimate intention is to help accelerate time to market and to develop robust processes.
Changing the Face of Continuous Manufacturing

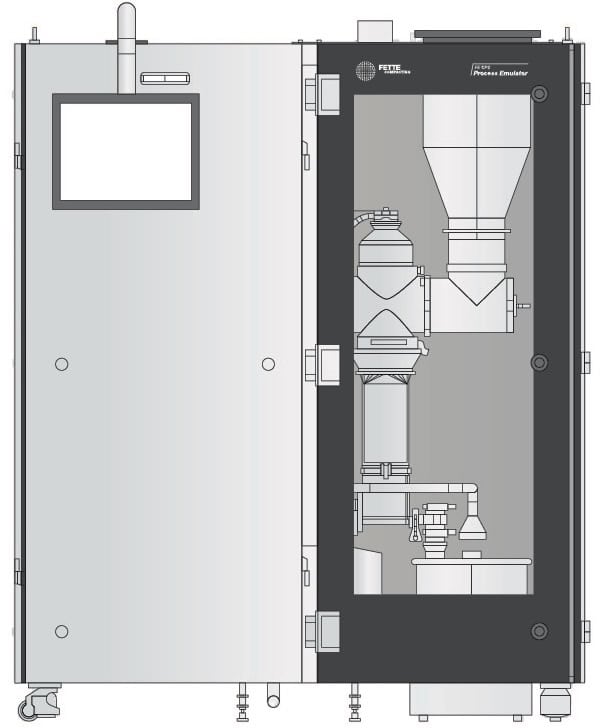
All of which brings us to Fette Compacting’s most recent, and novel, technological development: the FE CPS (Continuous Processing System). The FE CPS serves as a blueprint for the concept of process partnerships and as a catalyst for the idea of expanding those relationships to include process development. The pharmaceutical industry is currently undergoing significant changes, some of which are promoting a transformation towards continuous manufacturing: the product portfolios of original manufacturers are shifting considerably.
As a result of this dynamic, there is also growing motivation to look beyond the traditionally slow “batch” production model – after all, everyone wants to get their products to the market faster and become more agile and efficient, further supporting arguments in favor of continuous manufacturing. Many end users who are faced with making huge investments to replace aging equipment are seeing it as an opportunity to consider a wholesale shift of their own production philosophies. This presents a prime opportunity for the makers at Fette Compacting to offer their process knowledge and over 75 years of tablet press experience, as they continue expanding their new roles as consultants and service providers.
Thus the FE CPS is rapidly changing the way that some OSD manufacturers approach their own potential shift from batch manufacturing. The principle of direct compression enables the immediate transfer of the powder: it is transferred from the FE CPS directly to the tablet press via a flexible transport system. One of the most immediately recognizable features of the FE CPS is its compact size, which dramatically reduces the space-grabbing requirements plaguing earlier iterations of competitive technology. And it was designed for a wide range of application scenarios: it can process between 5, to over 200 kilograms of powder per hour.
The FE CPS is already proving efficient in the development phase: when compared to traditional batch manufacturing, the amount of material consumed can be reduced by up to 90 percent. Additionally, overall assessment time can potentially be brought down from three months to only one day – a dramatic reduction. These advantages are further exploited by the use of the aforementioned emulators, given how a product’s behavior on the emulator is exactly indicative of what will happen on the FE CPS (during its filling and dosing operations, for example).
ePAT – the Key to Real-time Release
In addition to the optimal interaction of all process steps, quality assurance and efficiency of continuous production depend largely on integrated analysis technology. This led to Fette Compacting’s development of the ePAT system, or embedded process analytical technology – a key component in the FE CPS. Also fully integrated into Fette Compacting’s tablet presses (especially when used in concert with the FE CPS), the sensors monitor crucial quality attributes at four strategic points: BU (Blend Uniformity) sensors are positioned at the outlet of the mixer in the FE CPS, in the hopper of the tablet press, and in the tablet press feeder. At the fourth position, a TU (Tablet Uniformity) sensor checks each individual finished tablet as it exits the die cavity just prior to final ejection. These real-time checks result in the immediate detection of quality deviations, allowing the user to make rapid processing adjustments and ensure overall quality.

The ePAT technology provides the foundation for real-time release testing, which represents one of continuous manufacturing’s biggest advantages. Continuous monitoring is of particular importance in product development given the opportunity to optimize the mixing process in a focused manner. The measurements are typically based on near-infrared spectroscopy (NIRS), which is a particularly efficient method. In the spectral range between 750 and 2,200 nanometers, NIRS detects a multitude of active ingredients without compromising the products, and allows for ultra-fast quality control while providing information on both the physical and chemical attributes of both powders and tablets.
The ultimate choice of measuring points is contingent upon the end user’s target objective. While it’s common in a development setting to employ several sensors plus an NIR-equipped inline measuring device (such as a Checkmaster), one or two strategically placed sensors typically prove adequate for continuous monitoring of critical attributes in a production setting. And the utilization of BU and TU sensors is not the sole province of continuous manufacturing; their benefits can also be gained in classic batch manufacturing production, given they can be used with tablet presses from both Fette Compacting’s FE Series and the updated versions of their renowned i Series.
An Alliance for the Ages
Recent successes demonstrate that Fette Compacting is already achieving its goal to prove a valuable partner for its customers in the early phases of product development using the FE CPS – even when it’s necessary to produce under strict regulatory requirements. A critical step towards the attainment of this goal is the now official collaboration with CMIC CMO USA, a well-respected contract manufacturer in the USA. The partnership between the two organizations sets new standards in pharmaceutical process and product development by combining innovative technologies with established procedural know-how.
A driving force behind the establishment of the synergy is the installation of an FE CPS unit into CMIC’s GMP-certified cleanrooms. This allows customers to benefit both from a shortening of typical timelines and from sound regulatory assurances. The ultimate goal of the alliance is to permit pharmaceutical studies and the development of new and optimized formulations, while an end user simultaneously tackles the challenges associated with potentially modifying or building new production space. Working with Fette Compacting and CMIC will allow all parties to streamline processes that had previously hindered the adoption of continuous manufacturing, and give the end user a powerful competitive advantage. This serves as yet another signal for how the FE CPS creates a strong link between strict quality standards and emerging, novel technology.
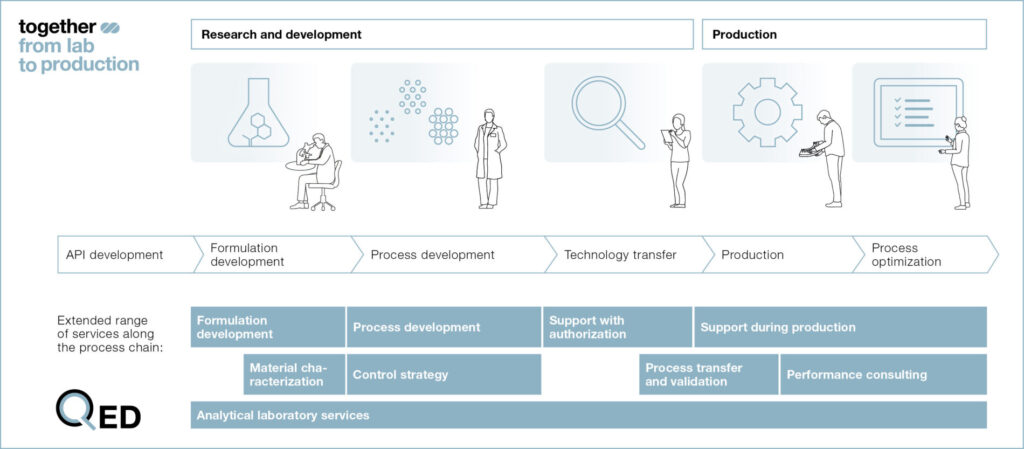
Finally, an integral part of the “Together — from Lab to Production” concept is Fette Compacting‘s global Customer Development Centers. It is at these locations that all available tablet technologies are maintained, with process experts on hand to assist our customers while they experiment with novel methodologies. There is even a complementary digital counterpart to this comprehensive process consulting: Fette Compacting’s Qualified Expert Database (QED). The QED represents an accumulation of experience gained from over seven decades of tablet press development and tableting expertise and is continuously updated with fresh insight. A strategic partner for its customers, indeed.