Fast, convenient, less perishable, instant foods have been around for about a hundred-fifty-five years, finding their origins in the necessity to solve social or economic problems. History is full of ideas and inventions to solve what people at that time called a problem. The first modern production process for dried milk was invented by Osip Krichevsky a Russian physician in 1802. The first vacuum drying process for powdered milk was patented in 1837. It is said that the powdered coffee idea was started by Nestle in the 1930s during the financial crash and the disruptions in the global coffee trade.
Brazil at the time had an excess of coffee beans, not knowing what to do with it, Nestle stepped in and offered to solve the problem. Nestle was already into powdered milk production and decided to apply the same evaporation spray tower method to the processed coffee beans and thus create e coffee powder. In 1938 the Nescafe coffee powder was created and instant coffee was born. Presently the instant coffee market has an estimated value of over USD 13 billion annually, which is a lot of instant powder.
Powder characteristics and morphology
Powders come in all shapes, forms, and sizes and while powder technology has advanced considerably over the past century the advancements diversify in all areas of industry and academia creating a deeper understanding of materials, their chemical make-up, and endless possible morphologies. This enables researchers and developers to improve product utility and pave the way for new technologies.
Powder characterization
One of the most common operations in the food industry is powder mixing, to create powder homogeneity for further application. Sounds simple and it is, in principle, yet it also comes with considerations. For example, a diversity of powders all have unique characteristics.
Food powders are used in everyday life and solve many food production issues such as shelf life, storage, transport, and ease of use. Creating food functional powders that cater to the desired consumer experience can be a complicated process when multiple food-related powders, minerals, and chemical additives are added together.
The different mixed powders will segregate differently, have different sizes, and flowability, have varying porosities, densities, unwanted agglomeration, etc. This powder characterization is not only defined by itself but also affected by the mixing methods, powder transition, processing times, equipment quality, design, etc. In short, the stability and desired use of the powder mixture is reliant on the particle knowledge, the powder characteristics, and the mixing process.
Fast dissolving powder benefits
While canned beverages ready to drink with the flip of a finger are readily available on every street corner, being convenient and easily portable, the market is open to the idea of having more options in the form of instant powdered drinks that are lighter by volume and packaged in easy to use sachets, tablets or tubs with a scoop. Isostar started this trend in 1977 with their powdered isotonic sports drink, since then innovations and cheaper production costs in freeze drying and powder production have sparked the interest to create more options when it comes to instant powder drinks aside from instant coffee, and chocolate. fruit and vegetable powders.
Powder technology allows for more flexibility when combining nutrients in powder form, increasing nutrient content, coating nutrients to prevent oxidation, and modifying particle characteristics to name but a few possibilities, that are a lot harder to accomplish with ready-to-drink beverages in a can.
Powder lumping
Fast premix powders offer many benefits in common food products for example cakes, gravies, soups, coffee, chocolates, and other beverages. But water, water vapor, humidity, and temperature are a powder’s worst enemy when stored, packaged, or surprisingly when it is desired to be rehydrated and mixed in a solution. Since each powder has different critical water activity values, moisture sorption isotherms can be used to determine those values and help ensure that powders are processed and stored below the powder’s specific values to avoid clumping.
It is also considered a limiting factor in microbial development, considering the values are below 0.6 aw. When Powder reconstitution and proper dissolution with water or other solutes is desired such as with a powdered sports drink, lumping can occur. A few possible factors can influence proper wettability, solubility, dissolution, the solution temperature usually water, glass transition properties (generally for grain-derived powders), the critical water activity, time, particle size, liquid bridges, hydrophobic particle properties, etc.
Liquid bridges are a common cause of powder caking during processing and storage or lumping during the reconstitution process. Particle porosity plays a key role whereby liquid bridges are being formed in-between the particles, creating temporary bonds that bind the particles together and thus forming lumps also known as fish eyes. These kinds of liquid bridges (bonds) are not very strong and basically, happen when particles are very small. The lumps can be limited with simple solutions such as stirring and warmer water but that does not add to the instant powder experience and defeats the purpose since time is needed to fully dissolve all the particles and you don’t want that with a sports drink at the gym because the dissolution rate of a post-mix powder is important for the user’s experience.
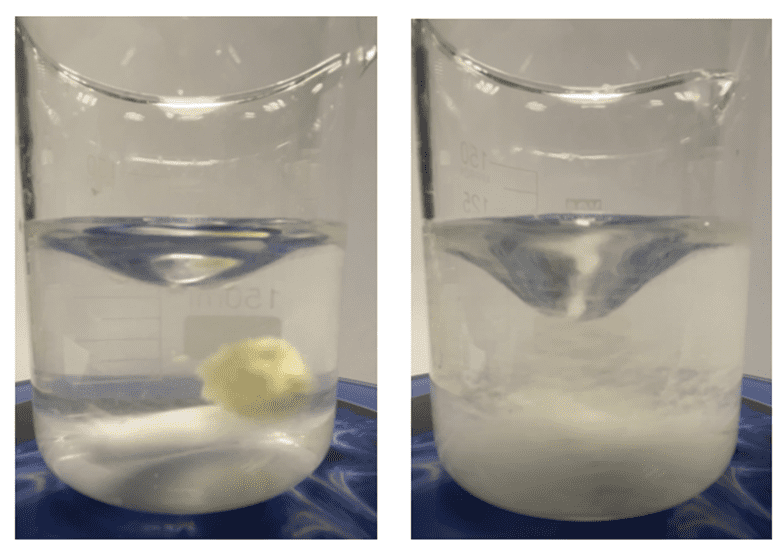
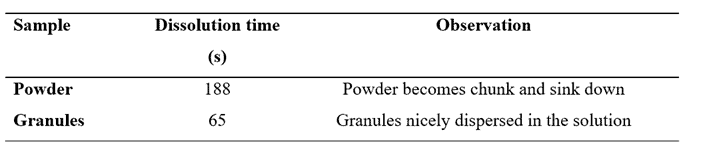
As an example, in a recent study conducted in our laboratory at DSS. An instant sports drink powder designed by iTapToo was tested. The powder exhibited problems during the reconstitution and dissolution phases showing the well-known lumping of part of the particle solutes. After having gone through client interviews and finally testing the powder, we found that particle size in this instant powder played a significant role in its partial powder lumping. When added to water the dissolution time was around 3 minutes, which is much too long for an instant sports powder product. As a solution a pelletizer was used to form larger granules, having more porosity and limiting the capillary forces of liquid bonds from forming, As a result, the dissolution time was cut down to 1 minute which was a first step success.
iTapToo wants to transform the market with a zero-waste philosophy to refill bottles with a healthy and delicious powder drink alternative to traditional soda beverages. An iTapToo refill point mixes the drink on-the-spot using a concentrated post-mix and adding filtered and chilled tap water.
This result shows that smaller powder does not always promise faster dissolution, therefore, other particle properties should also be taken into account.
This method is in-line with sustainability and offers customers a fast, easy-to-use post-mix drink before during, or after their workout. This is where proper and fast dissolution rates play a crucial role in the user experience.
As previously stated powders all react differently, especially when multiple food products and additives are mixed. That is why when designing and formulating a powder such as a pre-mix powder, it is essential to test the particle characteristics and take proper dissolution into account since it can hinder a product’s ease of use, but also have possible effects on non-mechanical caking and lumping during production, bulk storage or in packaging shelf life.