Silica, also known as silicon dioxide, is a naturally occurring mineral that has been used in various applications for millennia.
The Egyptians used it to preserve their mummies, while the Greeks and Romans used it to make glass. In 1824, the Swedish chemist Jöns Jacob Berzelius isolated silicon as an element for the first time and identified silica as its oxide. Shortly after in the 19th century, the French chemist Michel Eugène Chevreul known for his lipid research discovered that silica could be used as an anticaking agent in powdered products. In the mid-1800s, Chevreul was working for a French salt company that was experiencing problems with its salt clumping together during storage and transportation. He investigated the causes of the clumping and found that it was due to the presence of moisture, which caused the salt crystals to stick together. To solve this problem, Chevreul developed a method for treating the salt with small amounts of calcium silicate, which absorbed the moisture and prevented the salt from clumping.
This was the first practical application of an anti-caking agent, and it revolutionized the salt industry. Since then, the use of silica as an additive has become widespread in various industries due to its unique properties, including its ability to improve the flowability of powders, act as an anti-caking agent, and possesses high thermal stability, chemical resistance, and electrical insulation properties, among others.
Silica in Industry
Silica is used in various industries, including food/ beverage, agriculture, pharmaceuticals, cosmetics, construction, oil and gas, electronics, paint, coatings, and as an abrasive, polishing, and refractory material product to name a few. For example, in the food industry, silica ( E551) is commonly used as an anticaking agent and a flow agent to improve the flowability of powdered products and as a stabilizer in food and beverage products such as powdered milk, and spices.
In agriculture, it is used as a soil conditioner and as an ingredient in animal feed or as calcium silicate to improve the soil’s physical properties, such as its water-holding capacity and nutrient availability. This can help to improve crop yields and quality. Silica is also used as a foliar spray in some agricultural systems. When silica is applied in this way, it can help to strengthen plant cell walls and increase plant resistance to environmental stresses such as drought and high temperatures. Silica is also often added to animal feed as a source of dietary silicon, an essential nutrient for many animals such as poultry and swine. It can improve bone development, feather and hair quality, and these animals’ overall health and immune health function. In addition, silica can act as an anti-caking agent in animal feed, helping to prevent the feed from clumping and improving its flowability. One of the most common sources of silica in animal feed is diatomaceous earth, which is a sedimentary rock made up of the fossilized remains of diatoms, a type of algae.
Anticaking Agent and flowability
Globally Powders are commonly used in various industries due to their ease of handling, transport, and storage. However, powders can be challenging to handle and process due to their poor flowability. Silica has been found to improve the flowability of powders by reducing the interparticle forces that cause them to clump together. Silica acts as a lubricant, allowing the particles to move more freely, thus improving flowability.
The chemical process through which silica works as an anticaking agent is based on its physical properties. Silica is preferred due to its effectiveness to prevent powdered products from clumping together and its low relative cost. Anticaking is achieved by coating the powder particles with a thin layer of silica, which reduces the interparticle forces that cause the powder to clump together.
Silica is special because it is both hygroscopic and hydrophilic. Silica’s hygroscopic properties give it a strong affinity for water molecules and tend to absorb moisture from the surrounding environment. If silica was only hygroscopic as an additive in food products, the silica particles could adsorb moisture from the environment, which would lead to clumping and caking of the food product. This would be particularly problematic for powdered or granular food products, such as spices, sugar, and salt. At the same time, if silica was only hydrophilic it could adsorb water molecules onto its surface, which would also cause clumping and caking of powder products during processing and storage.
However because hydrophilic compounds like silica have an affinity for water molecules and can form hydrogen bonds on particle surfaces, they help to prevent clumping and caking in food products by reducing the attractive forces between food particles, in essence creating a barrier, this is aided by silicas high surface area to volume ratio which means that it can interact with a large number of water molecules.
Additionally, the Si-OH (silanol) groups on the surface of silica can form hydrogen bonds with water molecules, which helps to hold the moisture in place. The resulting coating formed by the silanol groups on the surface of the powder particles is also thought to have a lubricating effect, which further reduces the likelihood of clumping. This effect is due to the smooth and slippery nature of the silica coating, which helps to reduce the friction between the powder particles. Another benefit is that silica is also a non-reactive material, meaning it does not chemically interact with other substances. This property makes it ideal for use in powders especially food products because it does not alter the taste, texture, or other properties of the food.
Examples of silica use
Silica is used in a wide range of products, Its widespread use can be attributed to its effectiveness, low cost, and non-toxic nature. To name but a few, silica is widely used in powdered spices, cheese, milk powder, baking powder, instant coffee, beverages, paper, protein powder, mirrors, windows, bottles, concrete, bricks, and tiles. As well as toothpaste, deodorant, sunscreen, coffee creamer, tablets and capsules, computer chips, and optical fibers. sandpaper, metal polishers, paints, cat litter, water filtration, dental filling materials, denture adhesives, tires, golf balls, tennis racquets, missile components, thermal insulation materials, jewelry, abrasive cleaners, scouring pads, soil amendment, molding compounds, casting materials, pottery glazes to name a few applications in our daily lives where silica plays a crucial role.
Alternative silica additives
There are alternative additives that can be used in place of silica, such as talc, magnesium stearate, and calcium carbonate. However, these alternatives may not be as effective or as cost-efficient as silica. Tricalcium phosphate(TCP), Hydroxyapatite(HA), Talc, magnesium stearate, and calcium carbonate are commonly used as alternatives to silica in various applications. However, they are not as effective as silica and come with their pros and cons such as having good lubricating properties, improving powder flow, low cost, acting as a bulking agent, acting as a buffering agent to help maintain pH.
Silica usage in industries is still a viable and safe material to use until there is definitive proof of carcinogenic properties or a better material is found with the same beneficial characteristics.
Experimental results (Silica, TCP and HA)
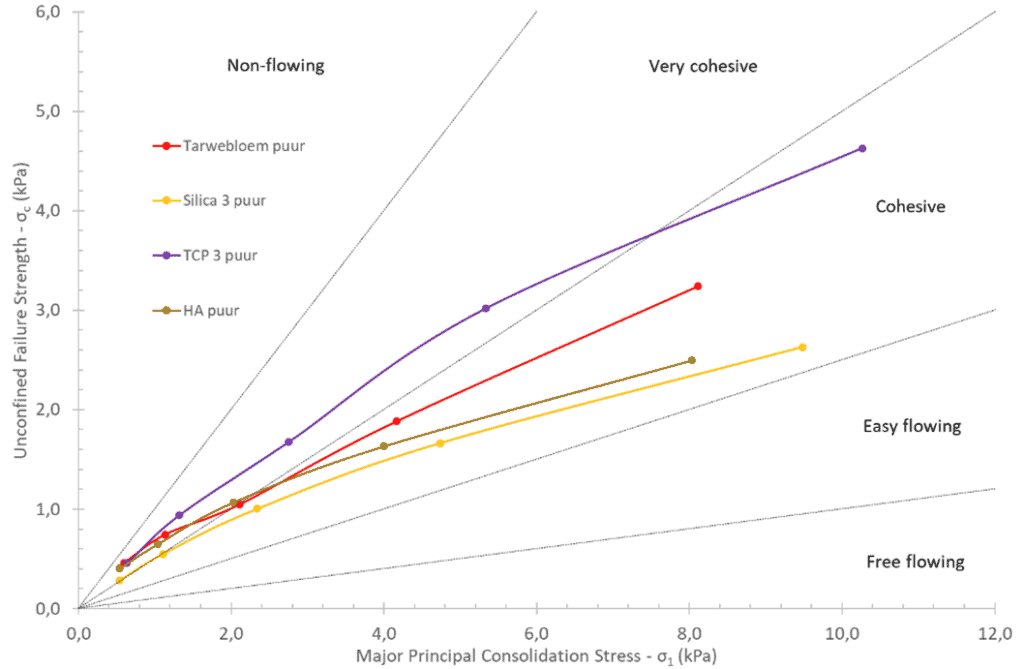
In Figure 1, the pure flowing agents and wheat flour are shown in the flow function of the shear cell powder flow tester and it can be seen that TCP 3 is more cohesive than the rest. This was also noticeable when working with the dust, where it often seemed to stick to walls, watch glasses, etc. It is logical that the flowing agents themselves are cohesive because the particles are so small and so many in number that intermolecular forces have a lot of influence. It should be noted, however, that silica 3 and HA are less cohesive than wheat flour at higher major consolidation stress.
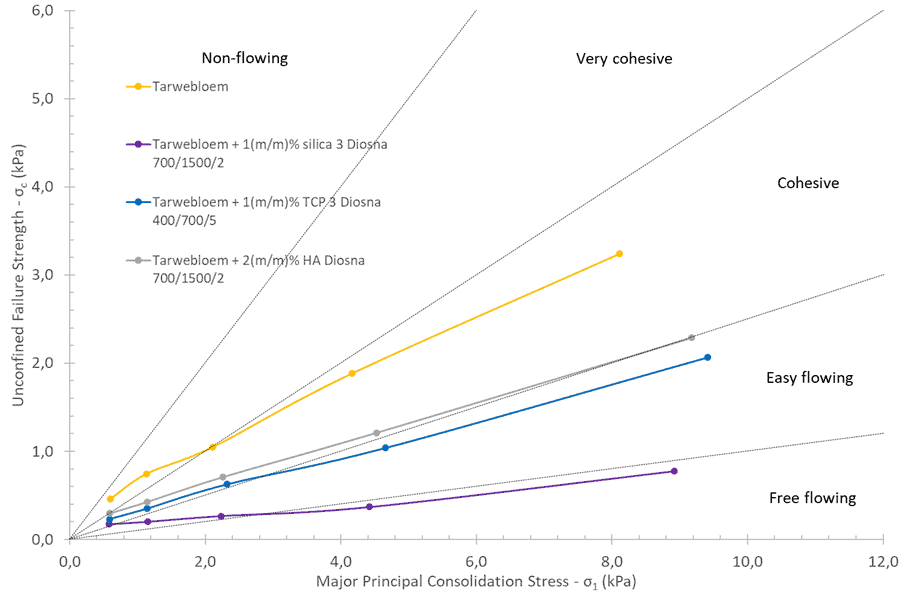
After the introduction of the flow agent in the wheat flour by using the high shear mixer, it shows clearly in the figure that the flow ability has been all improved. Silica is still the best flow agent among all three flow agents. The performance of HA and TCP is similar and both of them can improve the flow ability to a certain extent.
SEM results
It is clear that the flow agent attaches itself to the surface of the particles.



The small agent can then play a role as lubrication and cohesive particle can interact more smoothly.
Flowability testing methods
There are various laboratory testing methods for measuring flowability, such as the angle of repose, the Carr index, and the Hausner ratio. The angle of repose is a simple and widely used method that involves measuring the angle formed between a pile of powder and a flat surface. The Carr index and Hausner ratio are more complex methods that involve measuring the bulk density and tapped density of the powder. These methods provide information on the flowability and compressibility of powders, which are essential factors in the manufacturing and processing of powdered products. These methods provide information on the flowability and compressibility of powders, which are essential factors in the manufacturing and processing of powdered products. Delft Solids Solutions specializes in the analysis of powders and granulates, with a focus on the examination of porosity and particle size. The knowledge and expertise gained from these analyses are utilized to provide advice and guidance in the development of new methods and techniques related to powders. These services are available to a wide range of industries, including the pharmaceutical and food sectors.
In conclusion
Silica has been widely used as an additive in various industries due to its unique properties, including its ability to improve flowability, act as an anti-caking agent, and provide high thermal stability, chemical resistance, and electrical insulation properties.
However, there has been an increasing demand for silica alternatives, especially in the food industry, due to concerns over potential health risks associated with its use. Laboratory particle testing by Delft Solids Solutions has shown that some of these alternatives, such as magnesium trisilicate, calcium carbonate, and talc, can effectively replace silica as an anti-caking and flow agent in powdered products. However, silica is still the preferred anti-caking agent since it outperforms alternative additives in flowability characteristics. Although more research is needed to assess the long-term safety of these alternatives, they appear to be viable options for those looking to reduce their reliance on silica. Nevertheless, the decision to use silica or silica alternatives ultimately depends on the specific product and intended application. It is crucial to carefully consider each option’s potential benefits and risks.