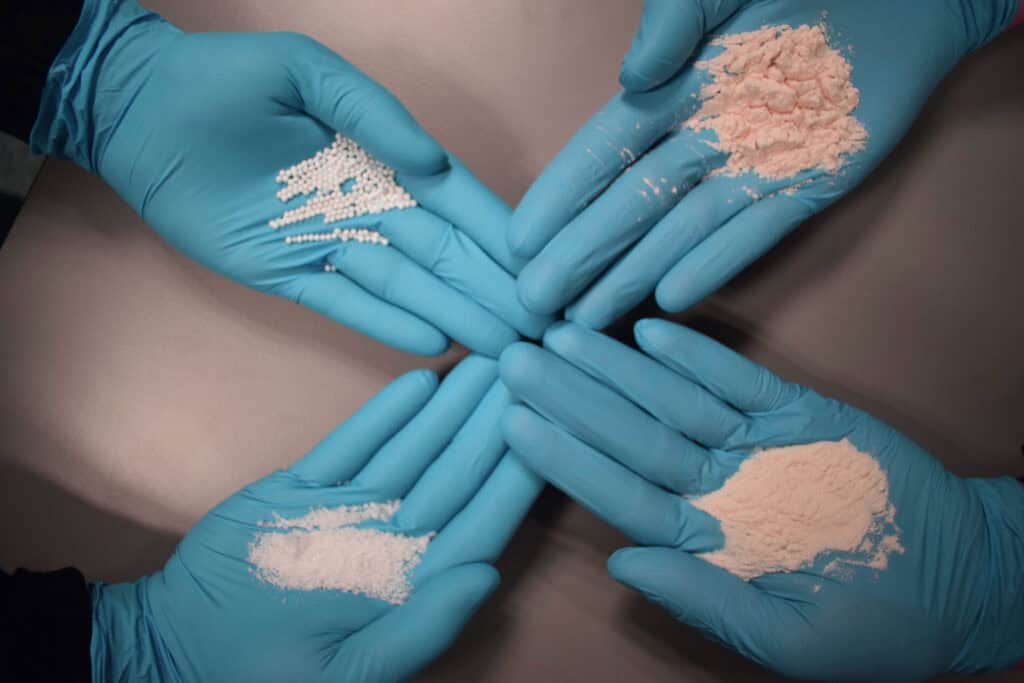
Proven in the formulation and dry mixing of powdered infant formula, adult nutritional products, and a variety of dietary supplements, the GMS Multiflux mixers integrate stainless steel construction in a smooth, hygienic design with the company’s proprietary Extractable Cantilevered Drive (ECD) system (optional) to promote compliance with highly stringent cleaning requirements. The Gericke ECD enables the entire mixing tool to be extracted from the housing on guide rails in seconds to fully expose the interior, invite complete cleaning, and assure the purity of the mixing process.
Installed in major nutritional product manufacturing facilities worldwide, the GMS Multiflux mixers feature a horizontal, double shaft and rotor design that gently combines multiple powders and liquids to meet the required recipe homogeneity within 30 seconds. A uniform mixture of base powders with probiotics, minerals, trace elements, flavors, and a variety of microingredients may be easily achieved without the excess shear and ingredient degradation common to traditional mixers.
The GMS Multiflux mixer line meets FDA and GMP requirements. Testing is offered in the company’s New Jersey test laboratory.