Direct compression is actually a simpler and more affordable alternative to this approach. However, this requires manufacturers to adapt their strictly defined, validated and documented procedures. The amount of time, money and administrative effort this entails can often discourage them from switching to the direct compression method. Nevertheless, as this article outlines, the time savings and cost savings made possible by the use of the Nauta mixer can make that extra effort worthwhile.

Today, the tablet is the most common dosage form for medicinal products. Pharmaceutical manufacturers must adhere to high quality standards and regulations to guarantee that each tablet contains exactly the right ratio of active pharmaceutical ingredient (API) in combination with the excipients such as binders, lubricants, flavourings or pigments.
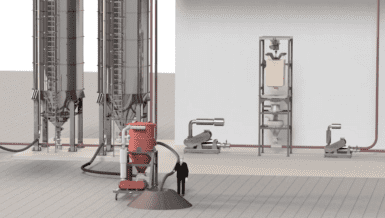
To achieve this, all the components must be optimally mixed before being pressed into tablet form. Many manufacturers mix the ingredients in a tumbler or container mixer and then perform either wet or dry granulation to reduce the risk of them separating again afterwards. However, as explained below, use of Hosokawa Micron’s Nauta mixer [Fig. 1] offers the best of both worlds. By combining direct compression with the avoidance of segregation, it can eliminate the need for granulation, thus saving pharmaceutical manufacturers time and money.
Wet and dry granulation
“In general, the risk of segregation is minimized by reducing the differences in bulk density and particle size distribution between the APIs and excipients,” says Bert Dekens, Application Manager in Team Pharma at Hosokawa Micron in Doetinchem, the Netherlands. “Manufacturers often implement a granulation step to reduce this risk.” Wet granulation involves multiple steps such as granulation, drying and screening. This makes it a time-consuming and hence cost-intensive process. Alternative dry granulation is used, especially when the product to be granulated is sensitive to moisture and heat. Although dry granulation is simpler and therefore less costly than wet granulation, it often produces a higher percentage of fine granules which can compromise the quality of the tablet.
Advantages of direct compression
A growing number of pharmaceutical manufacturers are discovering that direct compression offers a simpler and therefore cheaper alternative to these two basic granulation techniques. It entails the mixing of dry, free-flowing powders with a uniform particle size so that they can be directly compressed in a tablet press. For companies who are looking to eliminate the granulation step, Hosokawa Micron has the solution: the Nauta mixer. “Despite being so well known and having first been developed several decades ago, our Nauta conical screw mixer has been continuously improved in line with the very latest technological advances to keep it constantly up to date with the needs of customers in the pharma industry,” adds Dekens. “It is ideal for the low-shear mixing of delicate free-flowing powders. It enables all the necessary ingredients to be blended into a completely homogeneous mixture to ensure uniform and high-precision dosages. Moreover, the Nauta is hugely flexible in terms of the filling volume; it still produces an excellent mixing result even if the mixer is only 10% full. This also makes it suitable for use in a multi-staged mixing process to produce low-dose tablets.”
Total mixing solution

“At Hosokawa Micron, rather than just supplying machines, we take a broader approach to design a total mixing solution that integrates the pre-and post-processes too. [Fig. 2]
In other words, we always consider four aspects: how the powder is fed into the mixer, what happens inside the vessel, how the powder is discharged, and how the equipment is cleaned,” he continues.
“For the charging and discharging of toxic materials, for instance, we can use or develop customized container docking systems including lift systems or split butterfly valves [Fig. 3]. For the second aspect, during the mixing process itself, we take account of any ingredients the customer may need to add and how they will affect the process. This includes liquids, which can even be heated or cooled during mixing if necessary. Post-processing equipment can be integrated if required, such as deagglomeration equipment, sieves, cone mills and so on to turn the mixture back into powder.”

The conical vessel and top-driven agitation ensure that the powders will not segregate again during discharge into a tablet press, which can be a problem when container mixers are used.
The Nauta can even be positioned right above a tablet press if necessary, to ensure direct discharge which further contributes to maintaining the stability of the product.

The fourth aspect, cleaning, is extremely important in an industry such as pharma, which is why Hosokawa Micron offers complete automated cleaning in place (CIP) and sterilization in place (SIP) installations, including the associated control systems [Fig. 4].
“Our CIP and SIP skids remain permanently docked. Eliminating the handling step reduces the risk of product contamination and is also much better for operator safety in the case of toxic materials, for instance,” states Dekens. “Needless to say, all our systems comply with the applicable regulations and guidelines, and we provide the relevant documentation to prove it. That relieves the regulatory burden on our customers.”
The fourth aspect, cleaning, is extremely important in an industry such as pharma, which is why Hosokawa Micron offers complete automated cleaning in place (CIP) and sterilization in place (SIP) installations, including the associated control systems [Fig. 4]. “Our CIP and SIP skids remain permanently docked. Eliminating the handling step reduces the risk of product contamination and is also much better for operator safety in the case of toxic materials, for instance,” states Dekens. “Needless to say, all our systems comply with the applicable regulations and guidelines, and we provide the relevant documentation to prove it. That relieves the regulatory burden on our customers.”
Small-scale practical trials
Time is of the essence for manufacturers striving to win the race to market and secure the all-important patent for a new and original pharmaceutical product. To give them enough time to optimally explore the possibilities for saving costs by switching to direct compression, it is advisable for them to contact Hosokawa Micron as early on as possible – preferably during the R&D process. “After all, the formulation of each new tablet is unique, which is why we develop product-specific solutions together with each customer,” continues Dekens. “For instance, it could be beneficial to prepare the pre-mixes and subsequently add the excipients all in the same mixer, rather than splitting this up into individual process steps, and that’s precisely what the Nauta mixer facilitates. Our Test Centre in the Netherlands – which, thanks to its size and wide range of capabilities, is unique in Europe – enables us to work closely with customers to investigate such possibilities using the actual ingredients in real-life, small-scale trials.”
A second situation in which the direct compression approach makes sense, according to Dekens, is for generic pharmaceutical products: “These manufacturers have to focus on reducing costs, even if it means they have to redesign their production process, including revalidating their procedures as necessary. The cost savings they can achieve without compromising on quality make it an extremely worthwhile consideration.”
Long-standing experience

Hosokawa Micron has long-standing and proven experience of developing scalable technologies for the pharma industry, ranging from R&D to large-scale manufacturing set-ups.
Besides the Nauta mixer, the company also produces the Conical Paddle Mixer (CPM) for mid-shear mixing and the Cyclomix, which is widely used for the high-shear mixing of cohesive dry powder inhalers (DPIs) [Fig. 5].
“The required mixing energy depends on various factors. The powder characteristics such as the cohesion of materials and particle size are important, of course, but other considerations include the end-product properties as well as the process, safety and system requirements. Thanks to having so much experience and in-house technology, we’re a one-stop shop for mixing systems and can advise each customer about the best batch mixer solution – low, mid or high shear – for their particular situation,” he concludes.