Within this elaborate, complex production matrix that essentially operates 24/7, challenges are ever-present, one of the major challenges that have plagued the industry for decades is particle contamination. This contamination of particles is primarily due to poor mechanical design of conveying and processing equipment, equipment reliability issues, human factors, environment, transport, and storage. Particle contamination comes in a variety of forms, some you cannot see and others which you can see. The key is to remove the contaminants as close as possible to the source of origination or preferably prevent it from happening altogether.
Preventing those particulate/ product contaminations is therefore key in industries that produce consumable products, such as in the food, feed, and pharmaceutical production industries which, also other than downtime or damage to machines and parts can keep companies far away from expensive lawsuits.
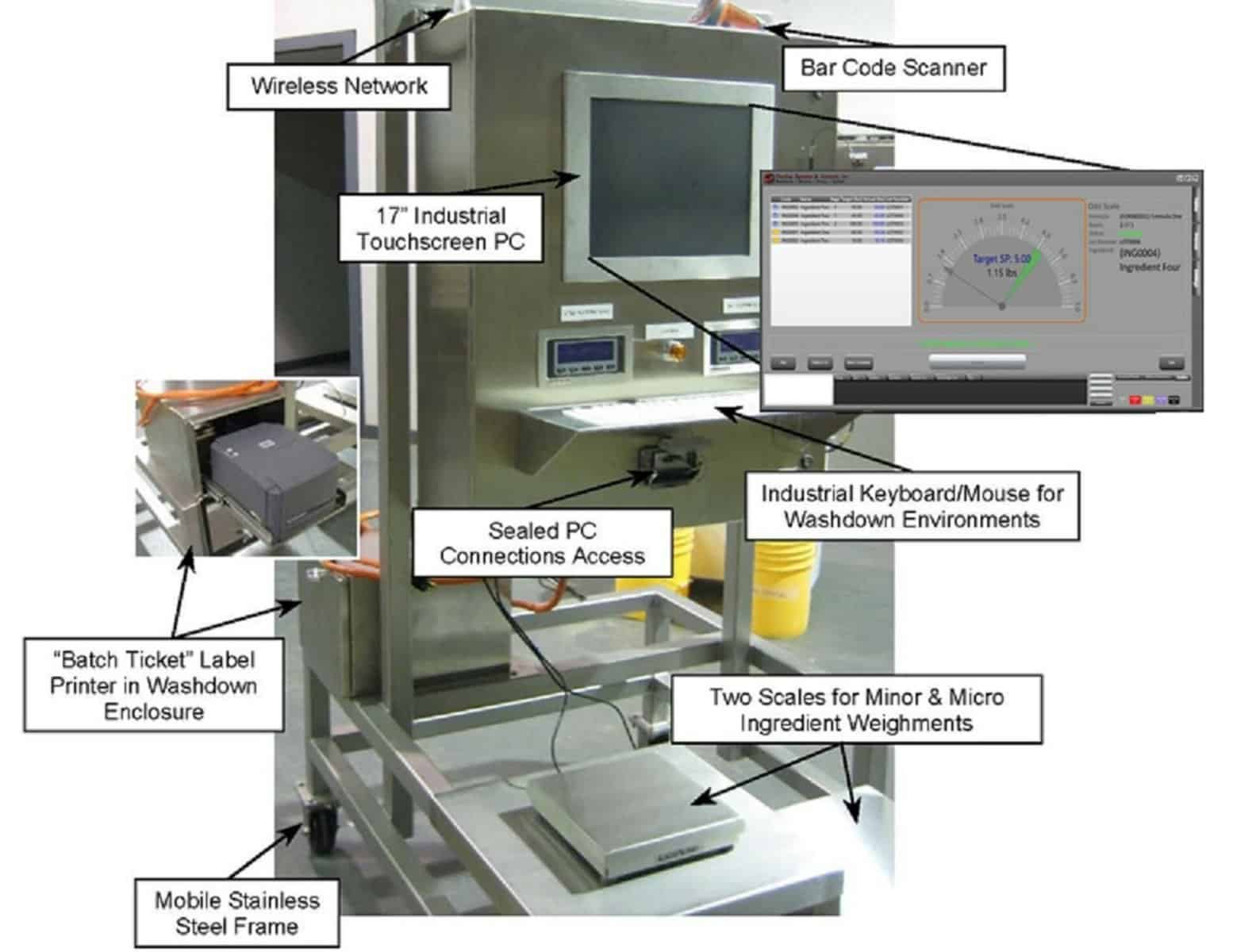
Air filtration systems and compressors
For example, the quality of compressor air drawn in from the surrounding atmosphere has a profound impact on the conveyed material and can introduce undesired foreign particulates such as dust and water from the moisture in the air. In industrial areas there are roughly 180 million particles in every cubic meter of air ranging in size from 50 microns to 100 nanometers, the majority of these suspended particles are too small for air compressor filters meaning they enter the conveying system. Proper use of filters both in correct filters and timely filter cleaning or replacement prevents contaminated air from interfacing with components and the conveyed material.
This helps to decrease component failure and increases system availability and reduced downtime. Regardless of how well a system is designed or how expensive it is, contamination will affect your system and product in varying degrees, therefore the system gas must be conditioned and decontaminated as much as possible with proper filters. Knowing the exact size distribution of the contaminant particles enables a more effective filter design and usage.
Another problem is water vapor, generally, a small compressor generating 100 cubic feet per minute (CFM) of air displacement would produce about 10,000 liters a year in average climate conditions. These aerosols and water vapor will also cause wear and tear of components, by affecting seals, and or lubrication, resulting in contamination such as pneumatic system oil (through valves and seals), rust, and the aforementioned dust and dirt particles found in suspension within the water vapor that was forced into the system.
These contaminants eventually end up in the product if proper filtration measures are not met. Air filtration does not end with compressors but is also paramount in HVAC systems and other areas during production where proper professional filtration equipment and separation are required to create the desired end product.
DSS testing
To maintain a system free of contaminants, filters, compressors, and carrier fluids must be monitored to safeguard the quality of semi-finished products and final products by regular sample testing. With modern equipment and techniques DSS can cover particle sizes from nanometers up to millimeters and investigate particle size distributions of dry powders or powders suspended in aqueous or organic liquids and even droplet size distributions. Image Analysis provides information on particle morphological aspects and dedicated particle counting techniques can be applied to determine the cleanliness of liquids. Nanoparticle assessment is established by electron microscopy approaches (both SEM and TEM), photon correlation spectroscopy, and analytical disc centrifugal methods. Chemical contamination by the small specimen is probed by SEM-EDX capable of identifying the morphology AND chemical identity of the respective contamination. Filter perfomance can be traced by porosity assesment employing mercury porosimetry
In this way DSS can cover the whole range of information needed for contamination research.
In some industries, for example, the pharmaceutical industry, the formation of nano dust particulates during medicine production is inevitable but poses a potential health hazard for the consumer.
Particles reduced to the nanoscale exhibit different properties than the same particles in the macroscale regardless if the particle was engineered or formed naturally as in the aforementioned dust.
These particles react differently to electrical conductivity and have different permeability characteristics.
In a 2017 study, it was found that the majority of vaccines were contaminated with nanoparticle-sized contaminants that were mostly metals. Therefore more research and testing need to be done, better HVAC air filtration systems, improved production environments, production procedures, equipment, and facility designs.
User error
Aside from equipment erosion, environment, and contamination, the most common cause of industrial particulate contamination is user error. Industry operators must ensure to adhere to production maintenance and sample testing protocols. Negligence for example in proper system installation, maintenance procedures, or repairs is always caused by user errors that can contaminate a production system. Another problem that is overlooked is that due to the growth of the particulate industry, chemical and processing engineers are being hired into areas outside their traditional fields of expertise leading to more possible user errors. Furthermore controlling particle contamination is rarely a part of a plant manager’s list of goals and focus. Other areas of contamination via clothing, gloves, improper decontamination protocols or procedures going from one part of the production facility to another area, and cross-contamination, especially in the food and pharmaceutical industries.
Storage Facilities
Most manufacturing plants have a storage system in place to hold material that is either waiting to be processed or has been processed and awaits subsequent transport. Even though there are many protocols and checks in place, dust, mice, molds, insects, and other foreign material can still find their way into the storage system and contaminate the material in some form or other. To preserve the safety of the end-user, these contaminants must be filtered out and prevented, for example in the mentioned industries trace amounts of halogenated anisole were found in consumer products and after laboratory testing, it was concluded that the cause was a wood-decaying fungi, found in wooden pallets which are widely used to transport products all over the world, in this case, drug packaging materials. The wooden pallets were treated with a fungicide, tribromophenol (TBP) a halogenated phenolic compound that is not used in western countries anymore but still enters the pallet market from other regions around the world. These pallets stored packaging material that got contaminated by the fungi which then contaminated the packaging and the end-user products resulting in an undesirable musty smell. Storage and transport are not exempt from contamination and undesirables can enter the production storage and supply chain at any given point.
Hence, particle science research and development Laboratories like Delft Solids Solutions with experience in particle science can help in finding the possible causes, give advice on improving system designs and look into new ways to improve the particle characteristics and their interactions within every stage of production.
Reliable storage
Reliable storage of bulk solids from silos, hoppers and big bags is essential. Investigation and testing of storage facilities can prevent or eliminate problems and is essential for quality control, material engineering, or the design of future equipment. Testing that can be done by DSS for transport and storage facilities are non-consolidated flowability and floodability according to Carr and consolidated Shear stress testing according to Jenike. Uni-axial compact strength testnig is used to to mimic potential caking in big bag and large storage containers as a function of load, time, humidity and temperature. During handling material can be spoiled or dusted and the dustiness potential of bulk materials can be research by a variety of methods ranging from Heubach to EN 15051 dust testing, where the latter provides quantitative determination of inhalable, thoracic and respirable dustiness fractions. Segregation finally is a phenomenon that often occurs during material movement being either filling or emptying a storage bin but also when transporting the respective material by e.g. trucks, pneumatic transport or belts and chutes.
Nanoparticle contamination
Nanoparticles differ from other particulates in that they are of a much smaller size and exhibit significantly different chemical, physical and toxicological properties. The application of nanotechnology in the industry offers many benefits but also poses potential risks to consumers and the environment because there is still insufficient knowledge of what the potential exposure to nanomaterials could pose in biological matrices and the possible future health effects. Many nano-oxide metals have very good catalytic properties whereby they can either increase or decreases chemical reactions that are undesirable, especially in a biological system and or an ecosystem, creating, for example, free radicals.
As society and technology advance, nano-technology and nanoparticle production both natural and synthetic will drastically increase. The impact of exposure to nanoparticles, both through the skin and via inhalation, is however fully unclear. These Nanoparticles may impact human health and ecosystems in ways that could be difficult to predict. As a result, the industry will have to become more adept at using nanotechnology to improve their products and their production systems while understanding their effect. For the latter purpose, new regulations and related analysis methodologies are being developed, which is followed closely by DSS in order to operate at the front line of nanotechnology and related regulations.
In conclusion
Quality control before, during, and after processing of particulates from their single raw unprocessed material to a final product is generally a complex procedure with countless areas where some foreign unknown particle contamination could enter the production stream. Holding production due to contamination found somewhere during the product processing is very costly and especially in the pharmaceutical industry avoiding contamination of foreign particles is a big concern since the stakes are exceptionally high when contaminants unintentionally enter and taint medication. These contaminants could lead to production downtime, extremely costly recalls, adverse side effects, possible deaths, and lawsuits, hence it is important to find the origin of the contamination as fast as possible and that is only feasible with due diligence and testing using sophisticated laboratory equipment and a state of the art testing facility.